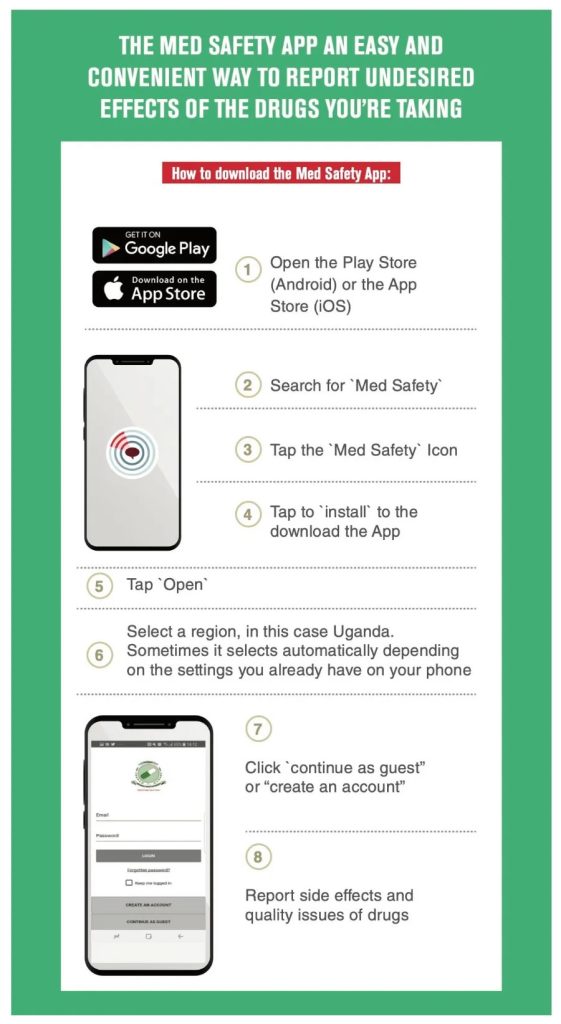
Report suspected side effects and safety concerns related to blood products (which are considered medicines) to the ZAMRA Safety Watch.
What is blood product?
Blood means whole human blood collected from a donor and processed either for transfusion or for further manufacturing, excluding blood specimens intended for pathology testing.
Side effects and adverse reactions from blood
A side effect is an unintended effect that happens in addition to the main intended effect (of blood). An adverse reaction is when the resulting effect causes harm (this can be both physical and mental).
There will be common side effects to you receiving blood, which your doctor or a healthcare professional will be able to advise on.
Safety concerns related to blood components
The Zambia Medicine Regulatory Authority (ZAMRA) also regulates human blood collected from a donor and processed either for transfusion or for further manufacturing, excluding blood specimens intended for pathology testing.
Healthcare professionals can report side effects of blood through the ZAMRA Safety Watch.
Why report side effects to the ZAMRA Safety Watch?
Everyone has a different genetic makeup and therefore it is very difficult to predict whether an individual will experience a side effect. Whether you are a healthcare professional or a member of the public, you can help others by reporting side effects which you or your patients experience to the ZAMRA Safety Watch. Reports help us gain a better understanding of safety aspects of blood, it’s preparation or use and safeguard patients through safety monitoring.