Welcome
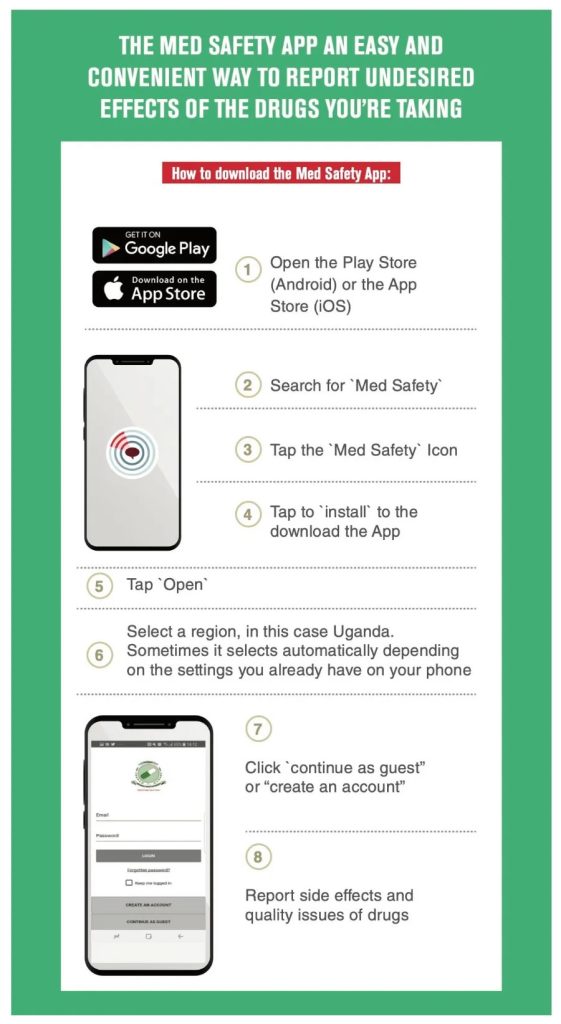
Health professionals, consumers and patients can voluntarily report observed or suspected side effects (adverse events, adverse reactions), product use errors and product quality problems for medical products to ZAMRA. Voluntary reporting can help ZAMRA identify unknown risk for approved medical products. Reporting can be done through our online reporting portal or by downloading, completing and then submitting the reporting form to Safety Watch: The ZAMRA Safety Information and Adverse Event Reporting Program.
Report Online